- California Assembly OKs highest minimum wage in nation
- S. Korea unveils first graphic cigarette warnings
- US joins with South Korea, Japan in bid to deter North Korea
- LPGA golfer Chun In-gee finally back in action
- S. Korea won’t be top seed in final World Cup qualification round
- US men’s soccer misses 2nd straight Olympics
- US back on track in qualifying with 4-0 win over Guatemala
- High-intensity workout injuries spawn cottage industry
- CDC expands range of Zika mosquitoes into parts of Northeast
- Who knew? ‘The Walking Dead’ is helping families connect
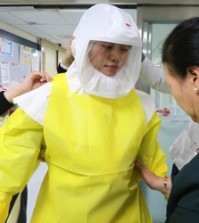
Experimental Ebola drug heals all 18 monkeys tested
(AP) — An experimental Ebola drug healed all 18 monkeys infected with the deadly virus in a study, boosting hopes that the treatment might help fight the outbreak raging through West Africa — once more of it can be made.
The monkeys were given the drug, ZMapp, three to five days after they were infected with the virus and when most were showing symptoms. That is several days later than any other experimental Ebola treatment tested so far.
The drug also completely protected six other monkeys given a slightly different version of it three days after infection in a pilot test. These two studies are the first monkey tests ever done on ZMapp.
“The level of improvement was utterly beyond my honest expectation,” said one study leader, Gary Kobinger of the Public Health Agency of Canada in Winnipeg.
“For animal data, it’s extremely impressive,” said Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, which had a role in the work.
It’s not known how well the drug would work in people, who can take up to 21 days to show symptoms and are not infected the way these monkeys were in a lab.
Several experts said it’s not possible to estimate a window of opportunity for treating people, but that it was encouraging that the animals recovered when treated even after advanced disease developed.
The study was published online Friday by the journal Nature.
ZMapp had never been tested in humans before two Americans aid workers who got Ebola while working in Africa were allowed to try it. The rest of the limited supply was given to five others.
There is no more ZMapp now, and once a new batch is ready, it still needs some basic tests before it can be tried again during the African outbreak, Fauci said. “We do need to know what the proper dose is” in people and that it’s safe, he said.
Ebola has killed more than 1,500 people this year and the World Health Organization says there could be as many as 20,000 cases before the outbreak is brought under control. On Friday, it spread to a fifth African country — Senegal, where a university student who traveled there from Guinea was being treated.
There is no approved vaccine or specific treatment, just supportive care to keep them hydrated and nourished. Efforts have focused on finding cases and tracking their contacts to limit the disease, which spreads through contact with blood and other fluids.
ZMapp is three antibodies that attach to cells infected with Ebola, helping the immune system kill them.
Of the seven people known to have been treated with ZMapp, two have died — a Liberian doctor and a Spanish priest. The priest received only one of three planned doses. The two Americans recovered, as have two Africans who received ZMapp in Liberia — a Congolese doctor and a Liberian physician’s assistant who were expected to be released from a treatment center on Saturday. A British nurse also got the drug, reportedly the two unused doses left over from treating the Spanish priest.
Doctors have said there is no way to know whether ZMapp made a difference or the survivors recovered on their own, as about 45 percent of people infected in this outbreak have.
ZMapp’s maker, Mapp Biopharmaceutical Inc., of San Diego, has said the small supply of the drug is now exhausted and that it will take several months to make more. The drug is grown in tobacco plants and was developed with U.S. government support.
Kobinger said it takes about a month to make 20 to 40 doses at a Kentucky plant where the drug is being produced. Officials have said they are looking at other facilities and other ways to ramp up production, and Kobinger said there were plans for a clinical trial to test ZMapp in people early next year.
The monkey study involved scientists from the Canada health agency, Mapp Biopharmaceutical, the U.S. National Institutes of Health and the United States Army Medical Research Institute of Infectious Disease.
Eighteen monkeys were given lethal amounts of Ebola in a shot, then received three intravenous doses of ZMapp, given three days apart starting three to five days after they were infected. Some were showing severe symptoms such as excessive bleeding, rashes and effects on their liver.
All treated with ZMapp survived; three other infected monkeys who did not get the drug died within eight days.
Primates have been good stand-ins for people for many viral diseases, but how well they predict human responses to Ebola, “we just don’t know,” said Dr. Cameron Wolfe, a Duke University infectious disease specialist. The study also “tells us nothing about side effects” people might have, he added.
Still, it was encouraging that even monkeys with severe symptoms got better, said Wolfe and Erica Ollmann Saphire, a Scripps Research Institute professor who has worked with some of the study leaders on antibodies to Ebola.
“The treatment window in humans needs to be established in a well-controlled trial” that also would explore the correct dose of ZMapp in people, Saphire wrote in an email. “Given its tremendous efficacy in the nonhuman primates, I don’t see how it couldn’t be helpful in people.”